We Develop. We Partner. We Innovate.

Ortholucent Technology
Improved visualization that enables clinicians to interpret bone healing with greater confidence leading to faster advancement of recovery protocols. We are revolutionizing orthopaedics beyond milling processing to mass produced additive manufactured implants and instruments.

Ankle Fracture Plating System
Apollo AFX is a comprehensive plating platform that provides a distinguishing clinical advantage by drastically improving intraoperative and postoperative visualization of bones and joint spaces. Plates are made using proprietary composite consisting of an additive manufactured (3D printing) titanium shell injection molded with a novel PEEK polymer (TI-PEEK).

Hip Fracture Nailing System
Artemis PFN is an intramedullary nailing platform for fixation of stable and unstable intertrochanteric and subtrochanteric fractures. It features an Ø11mm titanium alloy nail for robust strength, encased in an injection-molded carbon fiber reinforced PEEK material. This combination allows for subtle micro movements and promotes callus formation.

Cannulated Compression Screws
A comprehensive portfolio of Headed and Headless Ø2.5, 4.3, 5.6 and 7.4mm self-drilling and self-tapping screws that are cannulated for uncompromised precision. The radiotranslucent properties of the Creed screws provide a significant clinical advantage over traditional metal implants by drastically improving the visualization of bones and joint spaces.
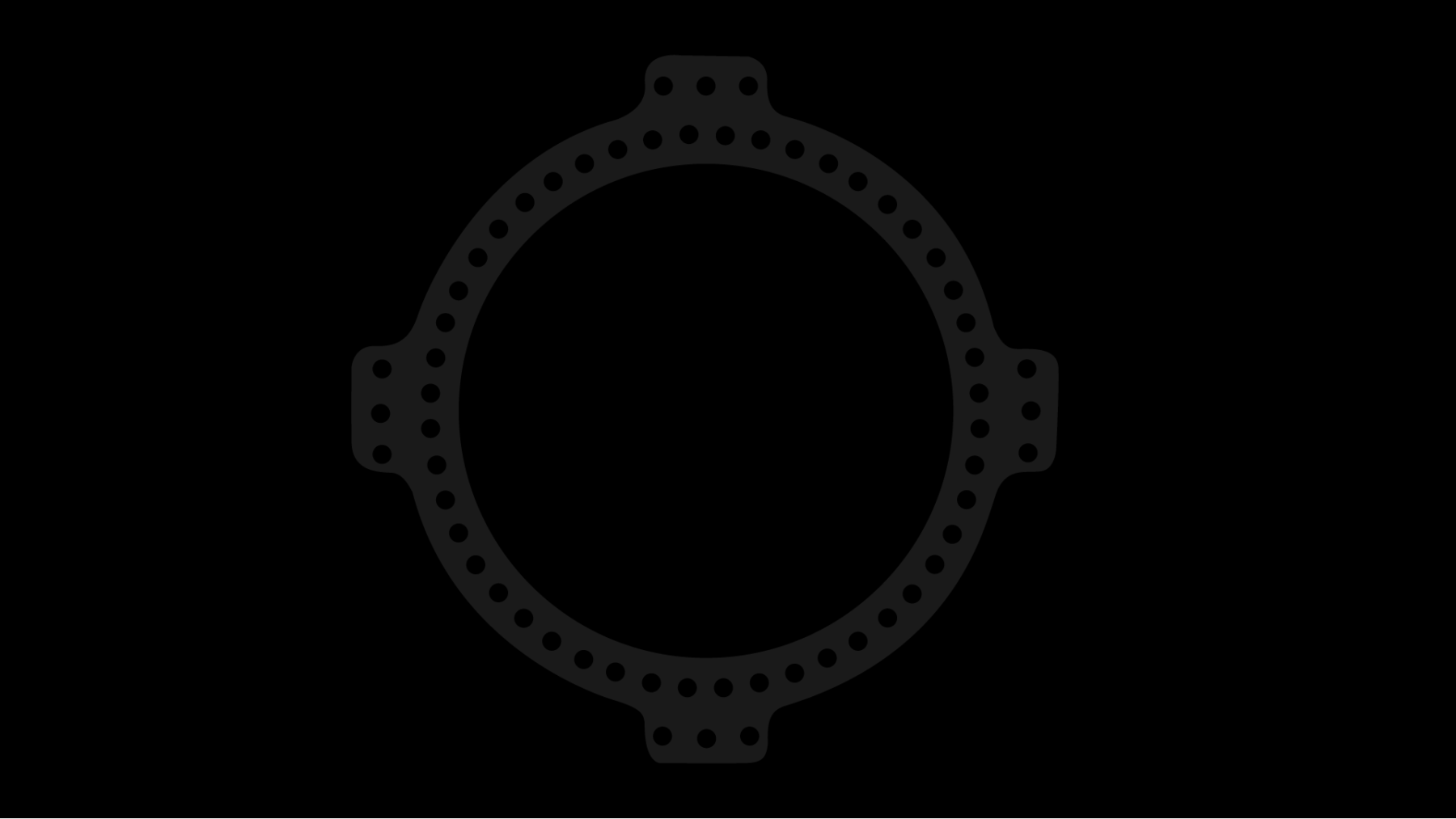
Circular Fixator
FusionFrame Ring Lock System is an updated Ilizarov Method circular external fixation apparatus, specifically engineered to address the extensive requirements of foot and ankle specialists.

Digital Fusion Implants
Cannulated HammerThread system is comprised of Creed Ø2.5mm implants and single-use disposable instruments, designed specifically for the correction of small bone deformities.
Contact
Thank you for your interest in contacting us. Browse the information below to find the best contact for your inquiry. Let’s create the future of surgery together.







