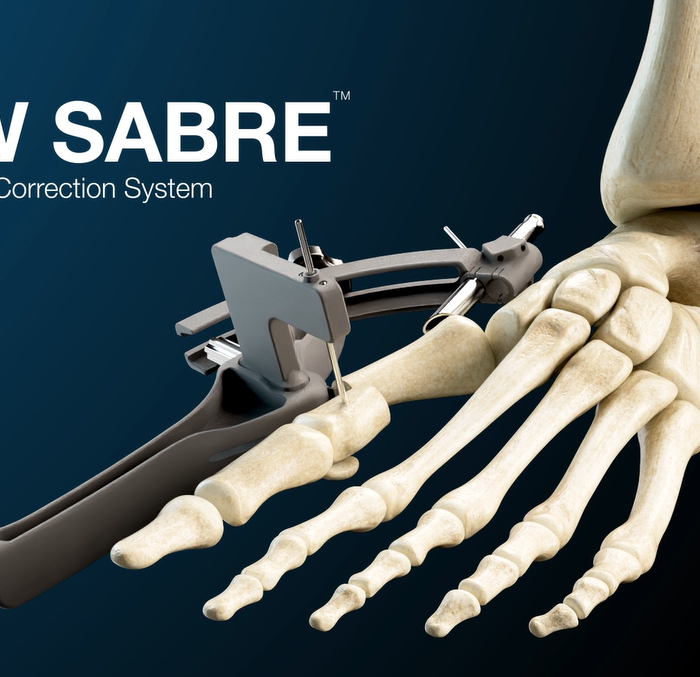
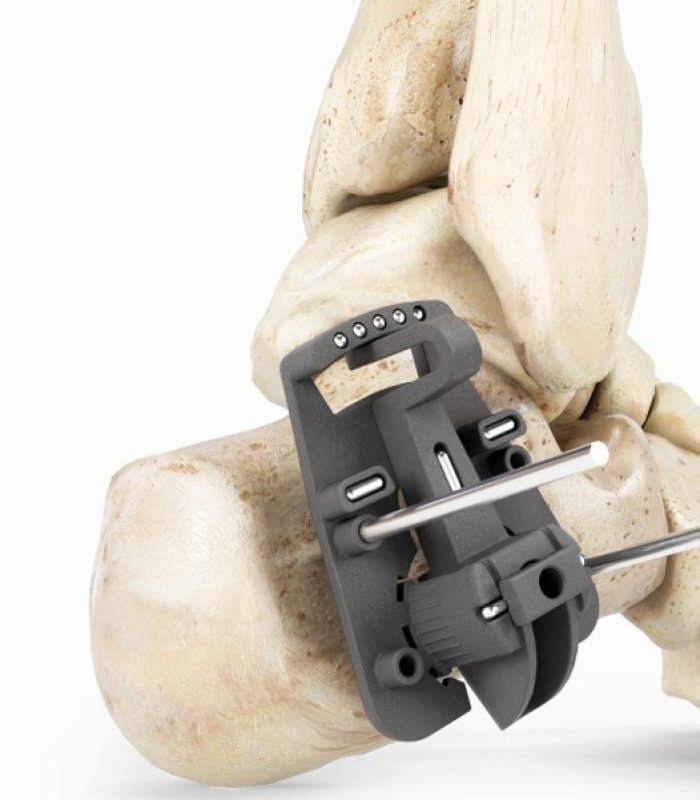
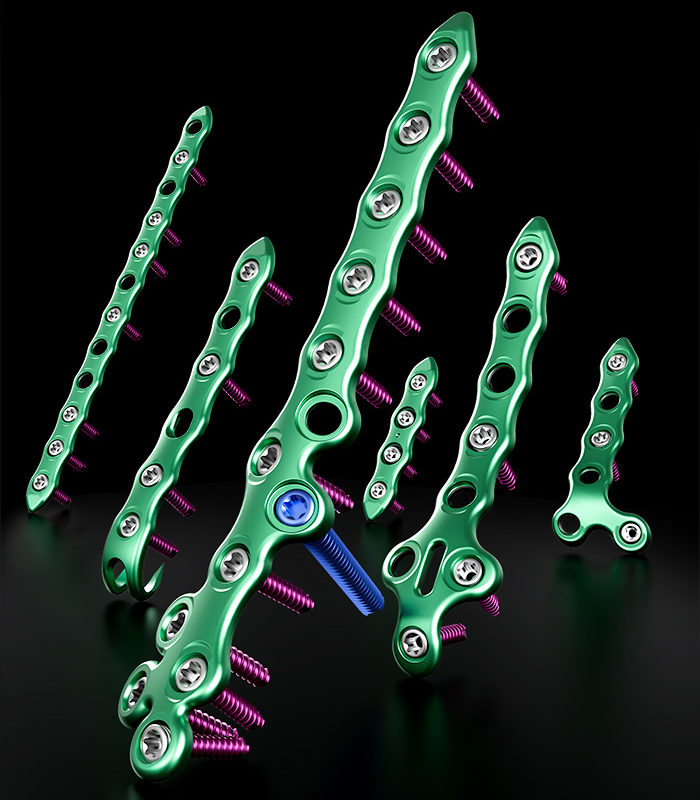
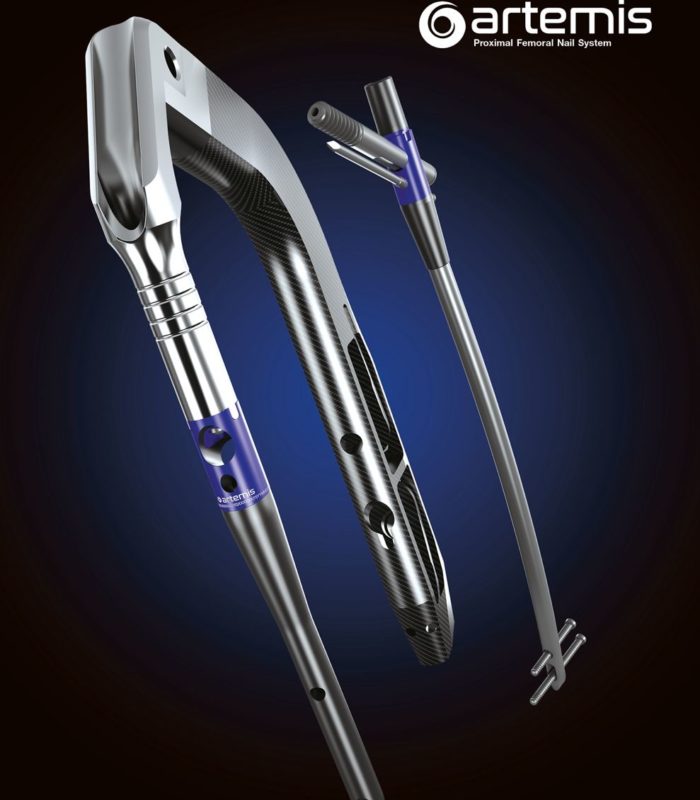
GLW Medical is a medical technology company focused on advancing orthopedic extremity and trauma surgery. We combine traditional and minimally invasive techniques with advanced manufacturing, including 3D printing and our proprietary Ortholucent Technology Platform, to create implants and instruments that enhance surgical efficiency and support faster patient recovery.
WE DEVELOP.
We engineer breakthrough solutions, leveraging advanced manufacturing to design, develop, and commercialize innovative orthopedic implants and instruments that set new standards in performance and reliability.
WE PARTNER.
We collaborate with surgeons through integrated R&D partnerships, transforming ideas into market-ready solutions and driving procedural innovation.
WE INNOVATE.
We own a growing portfolio of issued patents and pending applications covering hybrid materials and cost-effective manufacturing methods—pioneering the next generation of orthopedic solutions.
2015
2017
2021
2022
2023
2024
2025
2026

GLW, Inc. Founded
Research &
Development

CREED FDA 510(k)
Approval

FUSIONFRAME FDA 510(k)
Approval
ISO13485:2016 Certified
Commercialization
Early Product Release

ARTEMIS FDA 510(k)
Approval

APOLLO FDA 510(k)
Approval

Innov8ortho Partnership
Sales Distribution

CREED HAMMERTHREAD
Product Release

MDSAP Certified
Full Market Product Releases
Global Expansion

CREED EDGE
Product Release
CREED BURRS
Product Release

EXCALIBUR ZADEK
MIS System
Product Release
MEDICAL DEVICE DEVELOPMENT
World-Class Engineering
All startups companies have two enemies, cash burn and time. GLW has assembled a Research & Development (“R&D”) team which can, and has, “hit the ground running” resulting in projects moving faster than what is usual and customary in our industry. We look for ways to maximize speed to market without sacrificing quality or core competency. Our R&D team has the ability to evaluate a product strategy, frame up ideas and prototype concepts (working closely with design surgeons and internal engineering staff to gather feedback and design input), and then implements product solutions quickly and effectively.
Quality Management System
GLW believes that committing assets to the implementation and maintenance of a robust Quality Management System (“QMS”) is the way to “future-proof” our investment in a startup. We are MDSAP and ISO13485:2016 certified and conforming with FDA 21 CFR part 820 Quality System Regulations with particular focus on: 1) Design Control; 2) Risk Management; 3) Document Control & Records Management; and 4) Supplier Management. Our QMS is overseen by quality, regulatory, and legal professionals with decades of combined experience in medical devices.
Surgeon Centric Design Process
Design surgeons are an integral part of our design and development process. We assemble surgeon design teams specifically to each product and collect design and other product input from them. We identify the risks and concerns that surgeon design teams identify and then work systematically to mitigate those risks and address those concerns one after the next. Design surgeons are invaluable to our business strategy as they are the window into our future customer base. This approach, implementing design surgeon know-how from the start of the development process, is one of the key factors that makes investing in medical devices exciting. Engaging physicians throughout all phases of development, from design concept through prototype development and feasibility, and eventually marketing and educational strategies, allows a GLW startup to provide a laser-focused approach to streamlining the entire process of bringing a product to market. This has vastly improved the speed of development and has shortened the turnaround time of implant design, generation, and manufacture.
From Intellectual Property (“IP”) to Investable Intellectual Portfolio
We are passionate about our IP strategizing. Does the company have the freedom to operate in that field? What licenses might one need from third parties? We don’t waste money filing patent applications that are not essential to our product portfolio strategy. We restrict applications to those inventions that are worthwhile and those territories where there will have determined there will be a market or where we will be manufacturing. At the same time, we do not have tunnel-vision with respect to our IP strategy; we continuously consider and evaluate territories and markets where we might one day want to operate and do not limit ourselves only to those we are in today.
Scalable Manufacturing
A successful product design process must proactively consider and account for what is needed for the end goal of manufacturing. We create functional prototypes using design that contemplates at every juncture the relevant manufacturability practices. Investing in scalable manufacturing processes while reducing implant cost is at the core of GLW business model. Our innovative manufacturing processes do not restrict us in ways that traditional manufacturing currently does. This has given us significant degrees of freedom to allow for innovative designs that have never before been seen in the orthopedic market.
The Value Of Our Network
Engagement with end users is also very important to our product development process. Our team has an extensive collective network in the orthopedic and wider medical device marketplace including physicians, operators in manufacturing companies, and operators in distribution companies. Through our deep network, we can build a vast body of knowledge in a short period of time. Members of GLW have worked diligently to build their relationships within the industry over the course of decades. These relationships make it possible to address and resolve very complex problems simply by making a quick phone call and having an informal conversation with some of the best minds in the industry.
CREATING OPPORTUNITIES
Collaborate To Create
We are proud of our work with HCPs and have policies to ensure our interactions with HCPs are principled and appropriate. We invest in surgeon entrepreneurs with great ideas and provide them with direction and resources required to start bringing their product visions to life.
Inner Entrepreneur
Because of our unique background and expertise, we are able to develop, and commercialize products with a great amount of confidence and proven talent. We relentlessly support ambitions of all who dare to take on intelligent risk. We refuse to be bound by typical design and manufacturing constraints. Where solutions are not obvious, we maintain the freedom to explore until we find them.