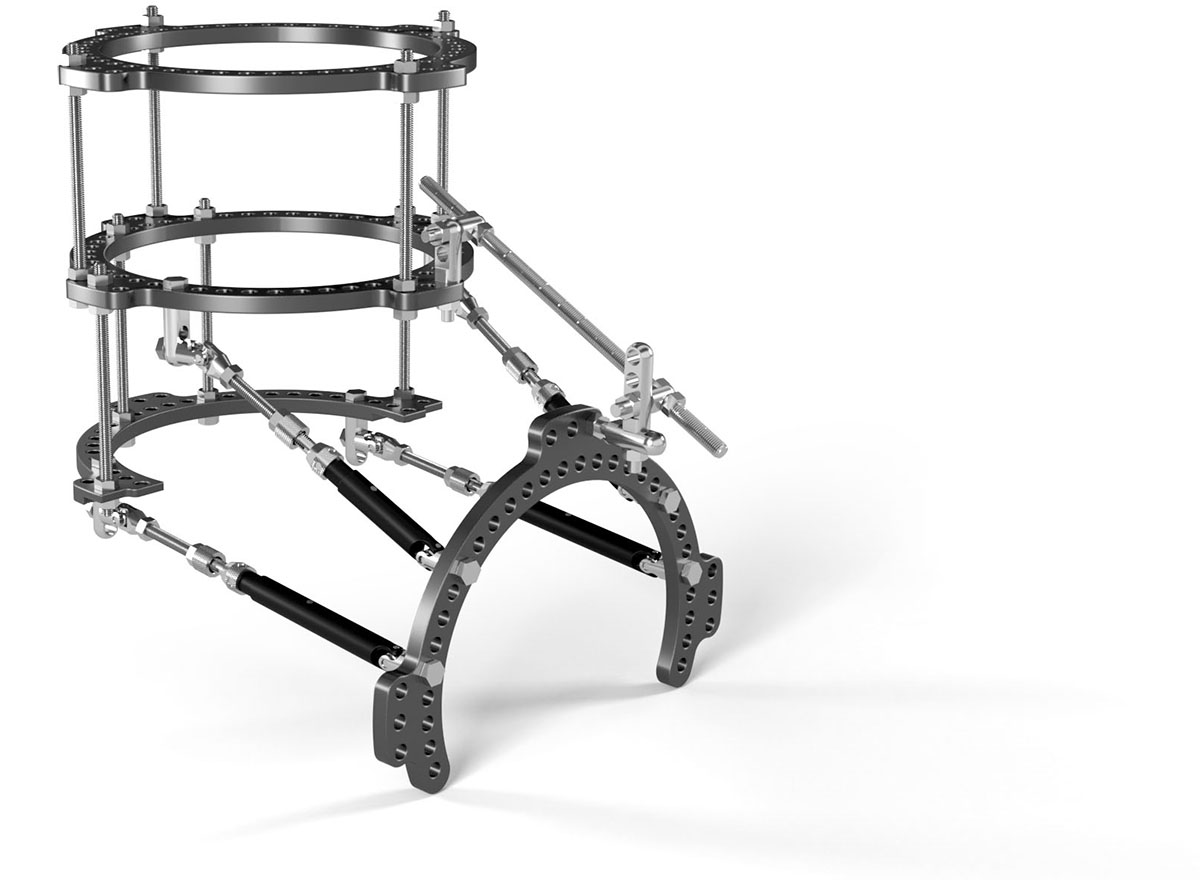
Carbon22 Consortium designed circular external fixator can treat complex foot and ankle deformities as well as provide a static frame for various indications.
ENGLEWOOD CLIFFS, NJ, USA, September 29, 2020 — GLW Foot & Ankle (Carbon22), a GLW Medical Innovation company announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new FusionFrame™ Ring Lock System, a circular external fixator. This versatile system was designed to be simple-to-use with a wide range of assembly components. FusionFrame™ was developed in part in collaboration with the Carbon22 Consortium, a select group of orthopedic and podiatric surgeons.
The unique design ensures circumferential access for precise fixation and can address a variety of indications in a straightforward and cost-effective manner. Multi-level constructs may be built to target the zone of pathology or apex of a deformity.
Axel Cremer, Chief Technology Officer for GLW, stated, “The 510(k) clearance for our FusionFrame™ Ring Lock System is an exciting milestone for Carbon22. Our world class team has done an excellent job of expanding our circular external fixator technology platform and building on the clinical success of the existing Ring Lock System. We’re excited to launch the FusionFrame™ Ring Lock System and look forward to introducing future variations which are currently in development.”
The FusionFrame™ Ring Lock System represents next-level functionality, utilizes state-of-the-art materials, and boasts an unparalleled design that provides the ability to customize frames specific to patient needs. This system can treat complex foot and ankle deformities, as well as provide a static frame for various indications.
“As we continue to develop our new, innovative patent-pending technology platforms, designed to allow for visualization of fusion sites (intraoperatively) and post-operative healing zones, we are working to ensure that foot and ankle surgeons have the right devices they need to treat today’s foot and ankle conditions,“ said Thomas H. Lee MD, Chief Medical Officer for Carbon22.
About GLW Medical Innovation
GLW is a medical technology company that uses its proprietary ortholucent technology platform to develop, additive manufacture, and sell orthopedic extremity and trauma implants and instruments. GLW owns issued patents and pending patent applications that cover hybrid materials and cost-effective manufacturing.
