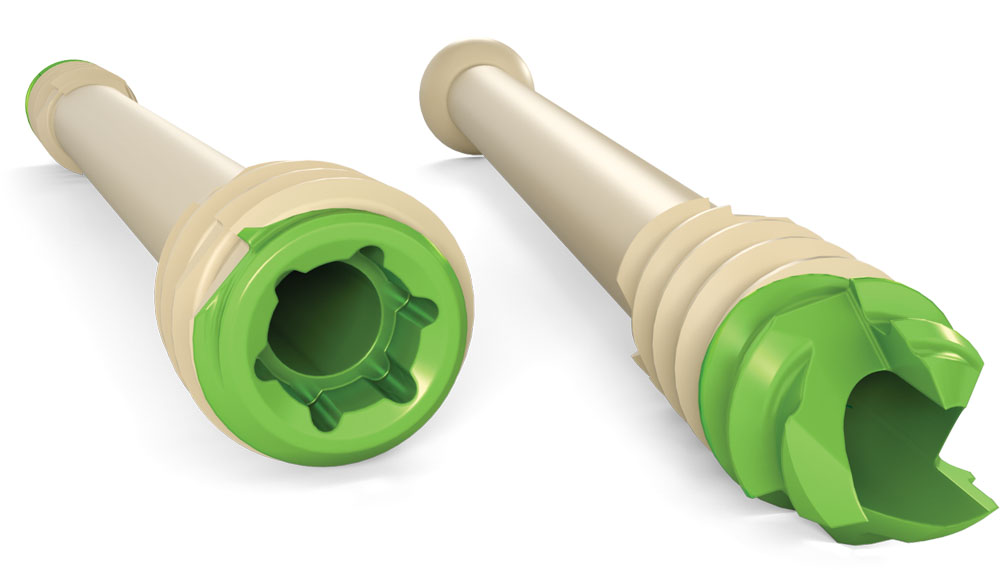
Englewood Cliffs, NJ – GLW, Inc. (“GLW Medical” or the “Company”), a medical technology company focused on advancing orthopedic extremity and trauma surgery, today announced that it has received 510(k) clearance from the U.S. Food & Drug Administration (FDA) to market the Creed™ Cannulated Screw System, a portfolio of unique, see-through “ortholucent” bone screws used for foot and ankle surgery. The Creed System was developed in collaboration with the Carbon22 consortium, a select group of orthopedic and podiatric surgeons.
The Creed system is a streamlined, multi-component platform featuring a distinctive, proprietary hybrid screw composite, consisting of a titanium core with an overmold of Solvay Zeniva® PEEK resin. GLW Medical’s ortholucent manufacturing technology offers several key benefits, including radiotransparency. While using medical imaging (both during and after surgery), the radiotranslucent properties of these novel headed and headless compression screws greatly improve visualization of bony structures. The Creed screws are offered in a comprehensive selection of diameters (2.5, 4.3, 5.6 and 7.4mm) that are available in a broad range of lengths.
The Company has created a bridge between materials science and device design, positively transforming the performance, manufacturing process and logistics of implant systems. These devices drastically improve cost-effectiveness, while maintaining, or improving patient outcomes.
“As surgeons, we continue to develop effective, skill-based surgical approaches to improve patient outcomes; however, device innovation has not kept up – until now,” noted Dr. Carroll P. Jones, director of the Foot and Ankle Fellowship Program at OrthoCarolina. “The Creed System is a pioneering device that provides a significant clinical advantage over traditional metal implants by radically improving intraoperative and postoperative visualization of bones and joint spaces.”
With a widened cannulation, Creed screws accept larger diameter k-wires and feature an enhanced self-drilling, self-tapping cutting screw tip that is unique to GLW Medical. Combined, these features help limit k-wire bending and reduce the amount of bone chips displaced by the screw, allowing the surgeon to implant with less effort.
Dr. A. Holly Johnson, orthopedic surgeon at New York City’s Hospital for Special Surgery, stated, “The Creed System is a long-awaited advancement in cannulated screw design. Company’s ortholucent technology has the potential to greatly improve our ability, as surgeons, to assess post-operative healing.”
Designed for maximum efficiency, the Creed screws and instruments are packaged sterile and surgery ready. Disposable instrument kits are available for all screw sizes, eliminating reprocessing costs. “Unlike surgical systems that are used repeatedly in hundreds of surgical procedures, Company’s Creed System ensures that every patient is treated with a sterile, single-use implant and new instruments functioning at their peak condition,” commented Axel Cremer, Chief Technology Officer for GLW Medical.
Dr. Lowell Weil Jr., CEO of the Weil Foot & Ankle Institute, emphasized that “GLW Medical has taken the next step by targeting its manufacturing processes and production costs, allowing it to provide the Creed System at the same price or lower than other manufacturers’ conventional titanium screws.”
This pricing flexibility will allow the company to aggressively penetrate and disrupt the U.S. foot & ankle implant market, estimated to be greater than $1 billion.
GLW Medical has an exclusive agreement with Solvay S.A. for the future development of medical-grade polymers.
ABOUT GLW MEDICAL
GLW Medical is a medical technology company focused on advancing orthopedic extremity and trauma surgery. By combining traditional and minimally invasive techniques with advanced manufacturing, including 3D printing and proprietary ortholucent technology, GLW Medical develops implants and instruments intended to support surgical efficiency and facilitate patient recovery.
