Press Releases
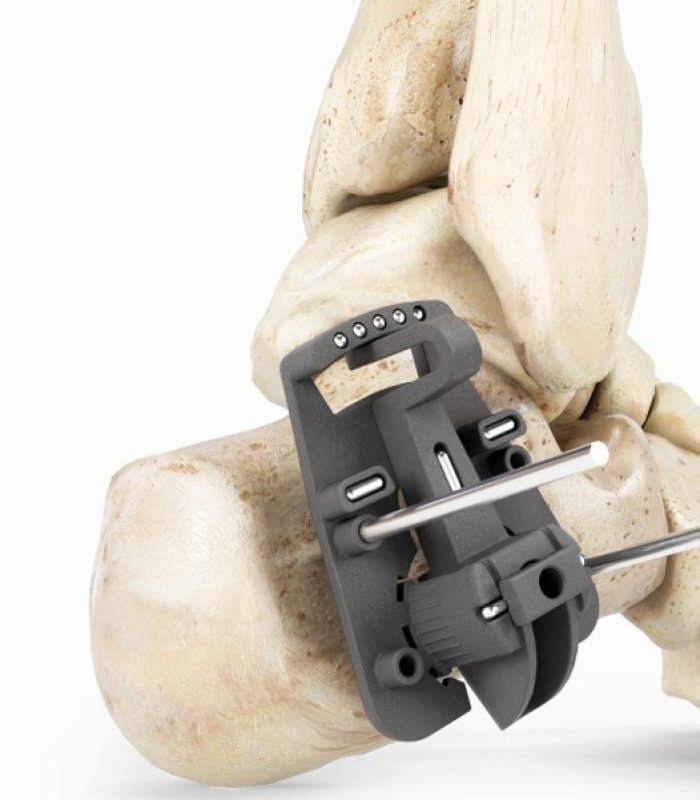
GLW Medical to Expand MIS Solutions and Introduce CREED Edge
Englewood Cliffs, NJ – GLW, Inc. (“GLW Medical” or the “Company”), a medical technology company focused on advancing orthopedic extremity and trauma surgery, today announced its upcoming product showcase at the 2025 American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting, taking place September 10–13 in Savannah, Georgia. GLW Medical will debut the CREED Edge Cannulated … Continued
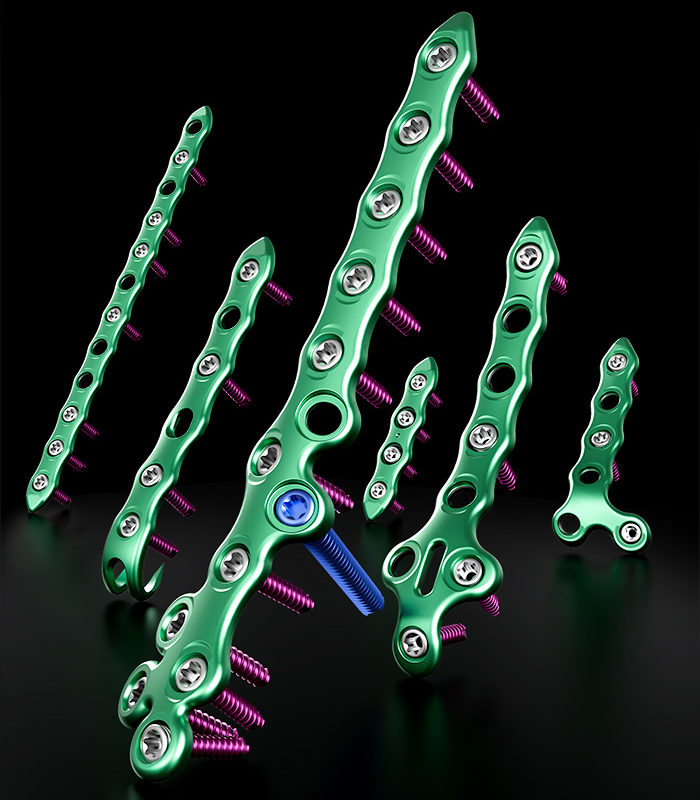
GLW Medical Announces Full Market Release of the Apollo Ankle Fracture Plating System
Englewood Cliffs, NJ., — GLW, Inc. (“GLW Medical” or the “Company”) with a focus on the foot and ankle orthopedic market is excited to announce the full market release of its Apollo Ankle Fracture (AFX) Plating System. The Company has commercialized its patented, first of its kind, additive manufactured titanium shell polyetheretherketone filled (TI-PEEK) plates … Continued
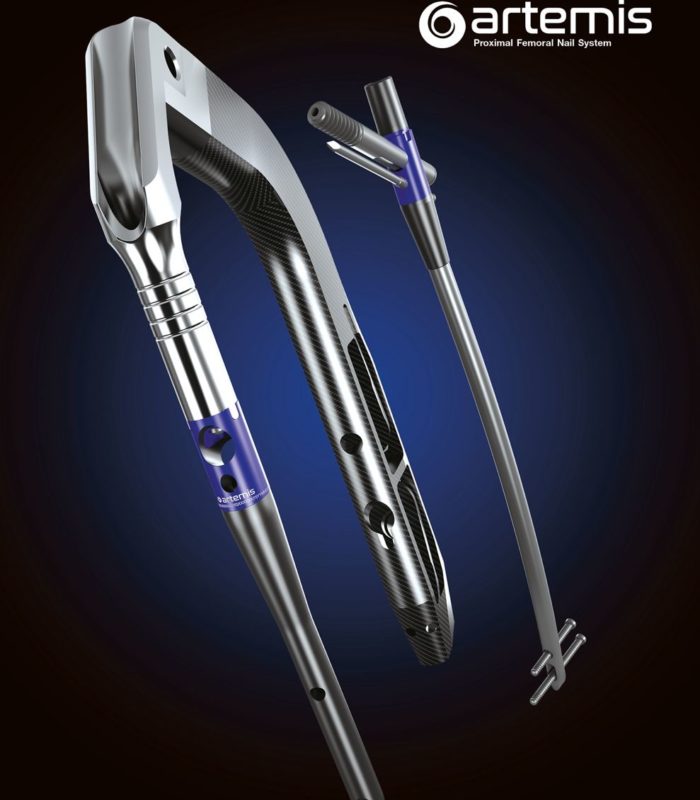
GLW Announces Additional FDA 510(K) Clearance for the Artemis Proximal Femoral Nail System
NEW LONG NAIL TO STRENGTHEN THE ARTEMIS SYSTEM AND STREAMLINE WORKFLOWS ENGLEWOOD CLIFFS, NJ, USA, October 31, 2022 / – GLW Trauma, a GLW Medical Innovation (GLW) company focused on a paradigm shift in the surgical treatment of trochanteric hip fractures, announced today an additional U.S. Food and Drug Administration (FDA) 510(k) clearance of the … Continued

GLW Receives FDA 510(k) Clearance for Apollo Ankle Fracture Plating System
Englewood Cliffs, NJ., — GLW Foot & Ankle (Carbon22), a GLW Medical Innovation company (GLW) with a focus on the foot and ankle orthopedic market, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its upcoming Apollo Ankle Fracture (AFX) Plating System, a novel portfolio of see-through, … Continued

Solvay and GLW partner on Creed™ Cannulated Compression Screws for orthopedics
Alpharetta, Ga., — Solvay’s Zeniva® polyetheretherketone (PEEK) resin was chosen by GLW Foot & Ankle (Carbon22), a GLW Medical Innovation company for its new Creed™ Cannulated Screw System for foot and ankle surgery. This advanced new screw system recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA). To create the new system’s ortholucent, plastic-metal hybrid … Continued
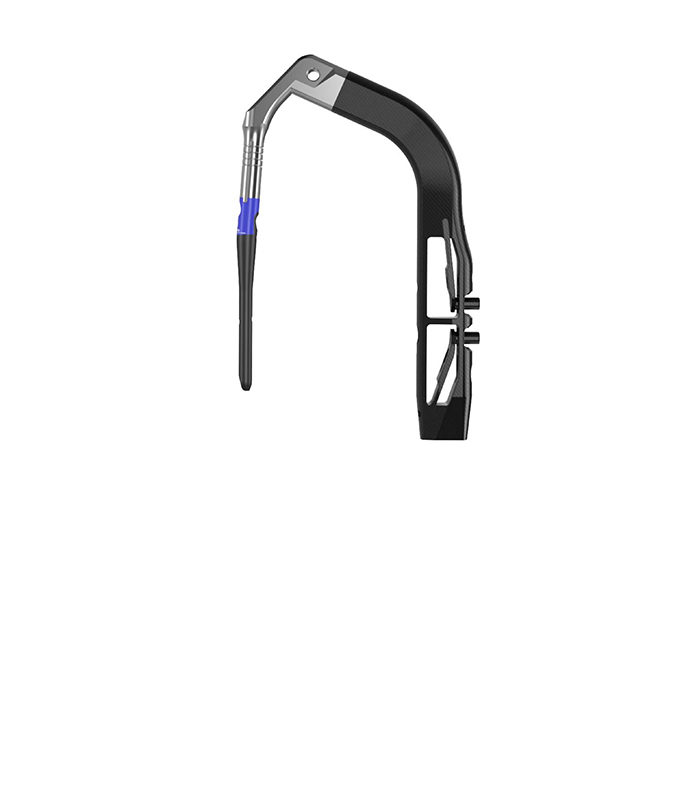
GLW Announces FDA 510(K) Clearance for the Artemis Proximal Femoral Nail System
A FOURTH-GENERATION HIP FRACTURE NAIL MANUFACTURED USING A REVOLUTIONARY PROCESS ENGLEWOOD CLIFFS, NJ, USA, February 22, 2021 / – GLW Trauma, a GLW Medical Innovation (GLW) company announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration for its Artemis Proximal Femoral Nail (PFN) System, a hip fracture nail. The … Continued

GLW Receives FDA 510(k) Clearance for the Creed™ Cannulated Screw System
ENGLEWOOD CLIFFS, NJ, USA, December 17, 2020 — GLW Foot & Ankle (Carbon22), a GLW Medical Innovation Company, announced today that it has received 510(k) clearance from the U.S. Food & Drug Administration (FDA) to market the Creed™ Cannulated Screw System, a portfolio of unique, see-through “ortholucent” bone screws used for foot and ankle surgery. … Continued
